Keywords
COLLAGEN + XENOGRAFT
-
Keywords
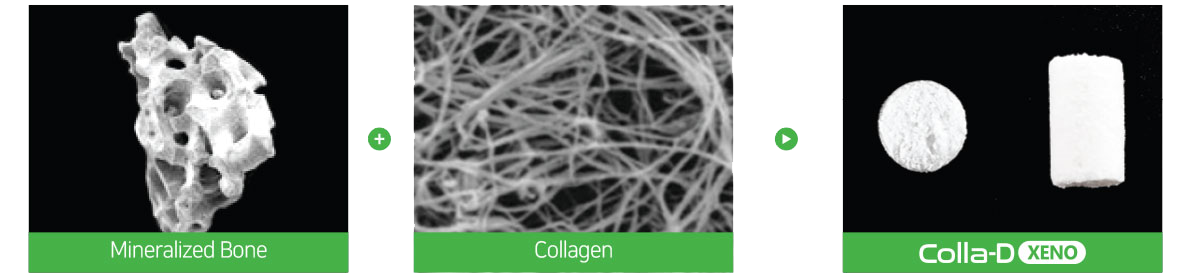
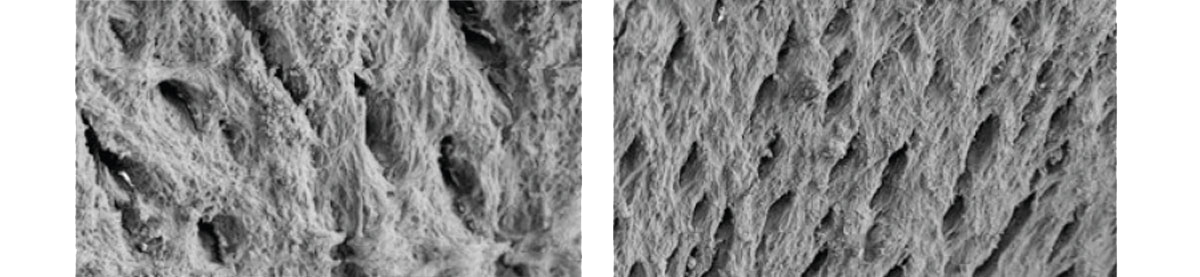
Creating a Favorable environment for bone regenerationEasy to useExcellent volume maintenanceHigh water absorptionHigh porosity of Bone-XP
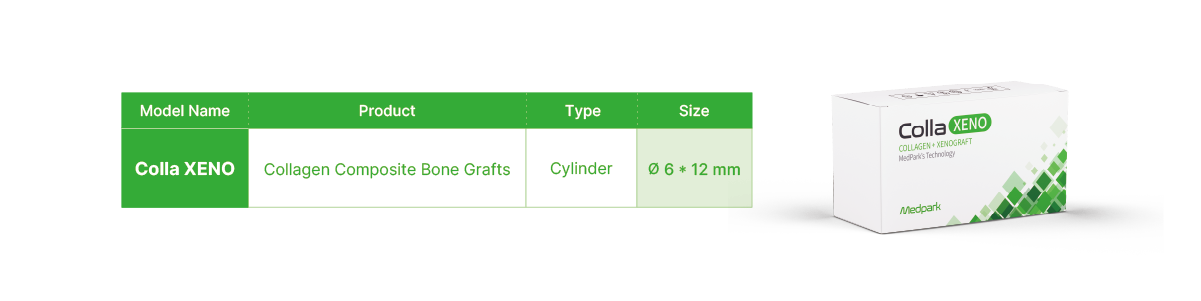
Technical Data
-
Intended Use
The bone defect is filled to induce regeneration of the damaged tooth tissue by dental disease or trauma, and applied to the local area for dental surgery.
-
Preoperative Preparation
1) Check the sterilization status through the sterilization C.I color attached to the product packaging. (Rad: good, yellow: bad)
2) Double-packaged products are kept sterile and moved to the operating room,
3) Firstly, check the packaging status of the product and do not use It If the Internal packaging is tom or opened.
4) Products that are expired should not be used.
5) Check the label and the actual substance of the product to see if it is suitable for use. 6) Before surgery, the medical team must read the instructions carefully for use of this product and understand the characteristics of the product and how to use it correctly. 7) Use this product as it is or hydrate it using purified water or saline solution. -
Directions for Use
1) After exposing the area of bone defect by elevating the mucoperiosteal flap, remove the remaining parenting tissue or other soft tissue completely.
2) Clean the surgical area so that there is no salive or contaminant.
3) This product can be used in a dry state or in a wet state with blood or physiological saline to be cut into a desired size with sterilized equipment
4) Use sterilized apparatus to implant this product to the defective area. Please avoid excessive filling. In order for this product to help the formation of the new bone,
it should be in contact with the surrounding bone in large area, and the peripheral bone or soft tissue should be rich in blood vessels.
5) When suturing the wounds, It is recommended that the periodontal tissue regenerating induction material is fully transplanted on the surgical area. and sealed in the surgical area.
*According to the judgment of the practitioner (or surgeon), the amount applied to the bone defect site and the period of healing can be controlled. -
Storage and Expiration date
How to keep and manage after use
1) Discard unused products after opening.
2) Do not reuse since it is disposable.
Storage method
It should be kept in shade or in a room temperature (1~30 °C) until use. -
Precautions
1. Warning
1) Do not re-sterilize or reuse since it is disposable.
2) Do not use it where physical loads are applied.
3) Do not overfill.
4) Make sure to supply sufficient blood on.
5) It should be used in areas rich in blood supply and in direct contact with bones.
6) Patient's tongue or saliva should not be leaked to prevent contamination.
7) Use it in an appropriate starile environment.
8) The product is released immediately before the start of treatment
2. Contraindications
1) Limit the use of the following patients or areas according to the doctors' clinical opinions. - lesions with osteomyelitis (part of chronic bone inflammation, such as osteomyelitis, or inflammation of surrounding soft tissue)
-Patients who have metabolic diseases
Diabetes, hyperthyroidism, osteoarthritis, connective tissue metabolism abnormalities and osteomalacia
-Patients with severe liver dysfunction and patients with severe kidney failures -Patients with steroid treatments (high-level corticosteroid treatments) -Patients who have local vessel abnormalities in the surgical araa
-Patients who have blood disease
-Patients who have collagen allergy symptoms
2) No effect on pediatric patients has been reported.
3) No affect on pregnant and nursing women has been reported.
3. General precautions
1) This product shall not be used for any other purpose then for inducing dental tissue regeneration
2) Before using this product, be sure to familiarize yourself with its usage and precautions. Failure to follow instructions for use and precautions for use may result in infection and side effects.
3) If side effects occur in patients who have received the transplant, immediately contact the manufacturer. The hospital and doctor who performed the transplant procedure shall take the necessary medical treatments for the patient.
4) After opening the package, it is recommended to use this product immediately and take care not to infect the product with external bacteria or microorganisms. 5) Do not use the package if it is damaged or expired.
6) This product shall not be used other than a trained dentist or specialist in the relative industry.
4. Side effects
1) Colla-Xand is a product containing collagen and may cause allergic reactions.
2) Periodontal surgery can result in the complications which are related with temperature sensitivity, gums twist, flap scab formation, ankylosed teeth and crestal bone loss, production of perforation or abscess, pain, swellings, inflammation, tooth decay, gingival transition and the use of anesthetic agents.
3) Depending on the degree and type of complications, the removal of the material or antibiotic treatment may be desirable by the judgment of the clinician.
Manufacturer
Manufactured by MedPark Co., Ltd. 24 Nakdang-daero 1570bean-gil, Bukçu, Busan, Tel. +82 61 301 8777
Sepcifications