Keywords
DEMINERALIZED BONE MATRIX
-
Keywords
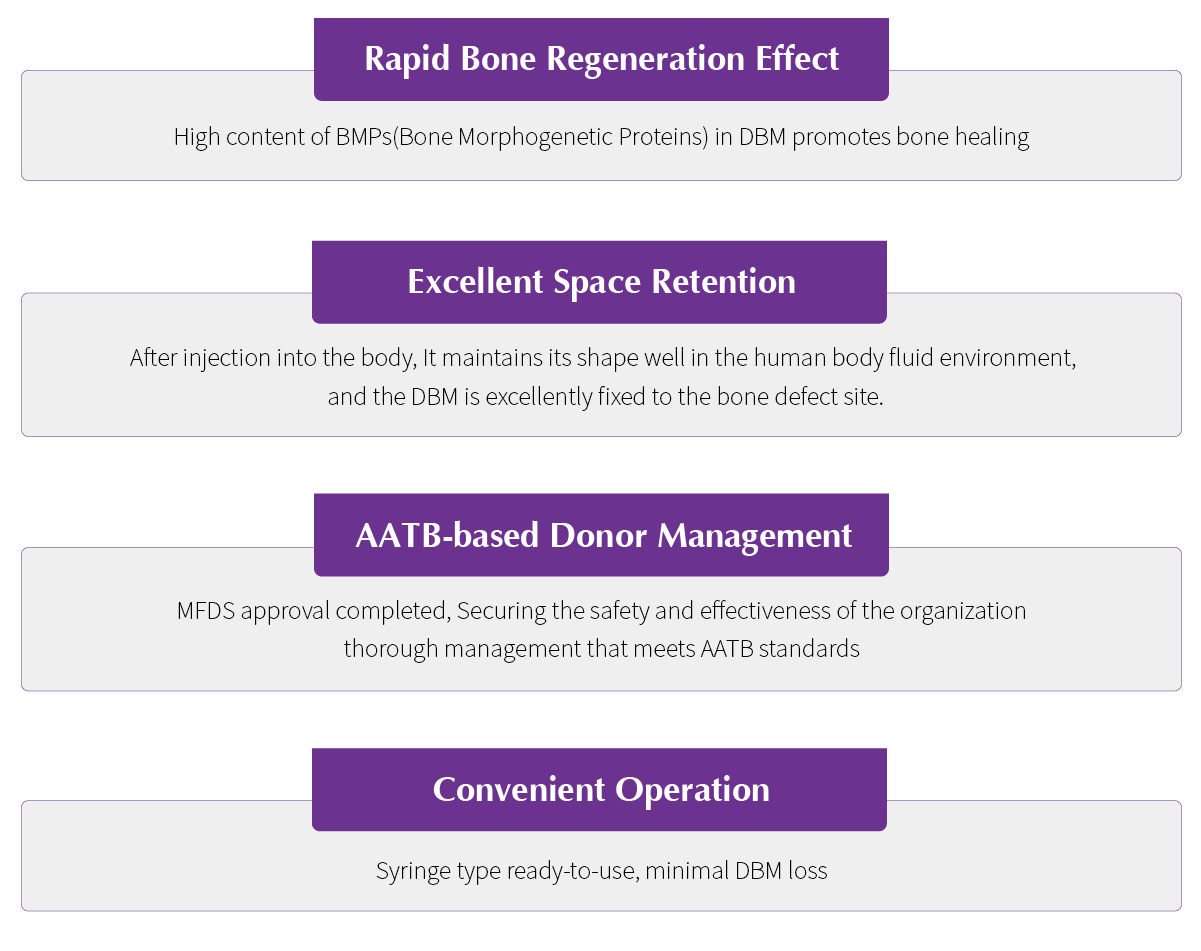
Rapid Bone Regeneration EffectExcellent Space RetentionAATB-based Donor ManagementConvenient Operation
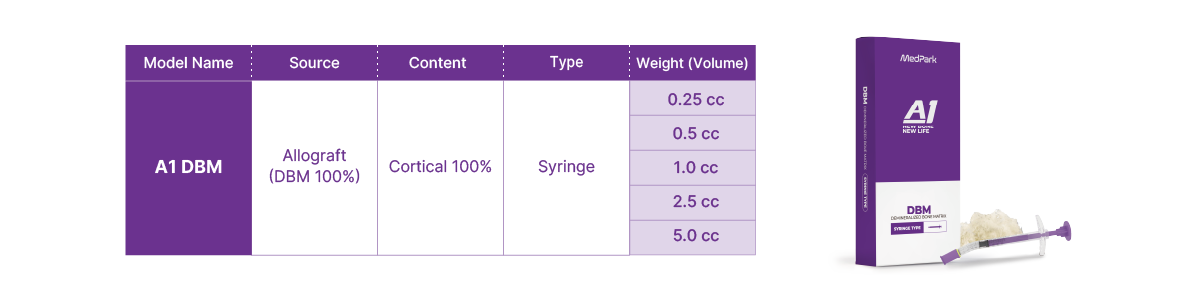
Technical Data
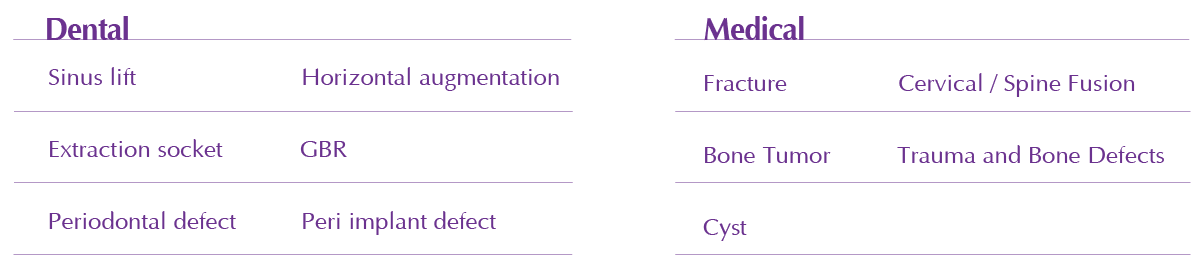
Sinus lift, Extraction socket, Periodontal defect, Fracture, Bone Tumor, Cyst
Sinus lift, Extraction socket, Periodontal defect, Fracture, Bone Tumor, Cyst
-
Intended Use
DBM (Demineralized Bone Matrix) is a biomaterial used for reconstruction of human bone tissue by stimulating the proliferation of mesenchymal stem cells, differentiation of osteogenic cells,
and promoting bone formation with osteoconduction and osteoinduction abilities.
-
Preoperative Preparation
1.Do not transplant at the site where is infected or an infection is in progress.
2. According to the Human Tissue Act, the tissue transplant result record must be delivered within one month after tissue transplantation.
3. The tissue should be used immediately after rehydration if possible.
4.Disposable, do not reuse.
5.Check the sterilized packaging before use.
6.Do not use a product that expiration date has passed.
7.If contaminated products are found, discard or return them.
8.It should be used by doctors and dentists, and use other than the intended use is prohibited.
-
Directions for Use
This product must be stored at room temperature (1~30℃, 34~86℉) and individually hydrated.
1. Vial Type
1) Open the outer packaging (Box packaging).
2) Remove the Tyvek packaging attached to the blister and take out the vial.
3) Check if the vial is damaged and remove the double cap.
4) Rehydrate the tissue in a container with a sterile solution stored at room temperature.
5) The tissue should be used as soon as possible after hydration.
2. Syringe Type
1) Open the outer packaging (box packaging) and take out the blister.
2) Remove the Tyvek pouch (secondary packaging) attached to the blister and take out the inner blister.
3) Remove the Tyvek pouch (primary packaging) attached to the blister and take out the syringe.
4) Remove the outer protective cap.
5) Apply to the area to be transplanted by pushing the syringe's pusher.
6) Fill in the bone defect area using a sterilized instrument.
-
Storage and Expiration date
This product can be stored for up to 3 years in an unopened package at a dry room temp(1~30℃, 34~86℉)avoiding direct sunlight.
-
Precautions
This product is produced from donated human tissue and should be used only once per patient.
Manufacturer
MedPark|#1005, #1008~1010, 25, Seonyu-ro 13-gil, Yeongdeungpo-gu,Seoul, Republic of Korea|TEL : 82 2 2257 8330
Sepcifications