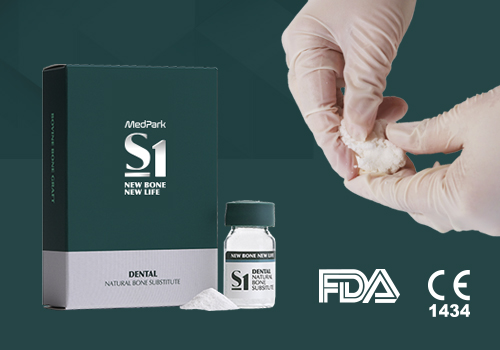
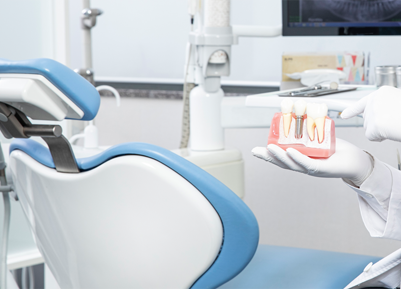
Regenerative medical solution provider
MedPark
VISION 2025
IPO
KOREA
No.1
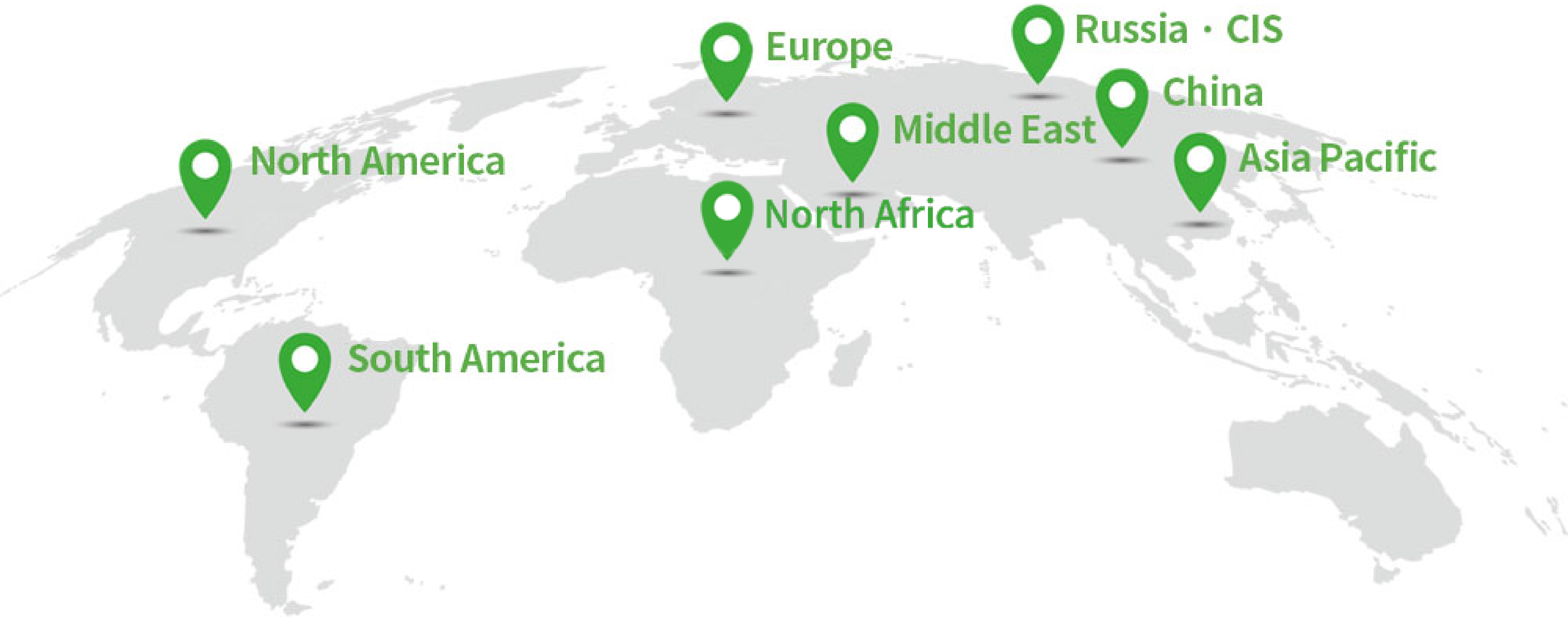
Asia-Pacific
No.1
Global
TOP10
First in Korea and Asia
Completed clinical and commercialization,
acquired global certification MedPark's dream is the future of regenerative medical.
acquired global certification MedPark's dream is the future of regenerative medical.