Keywords
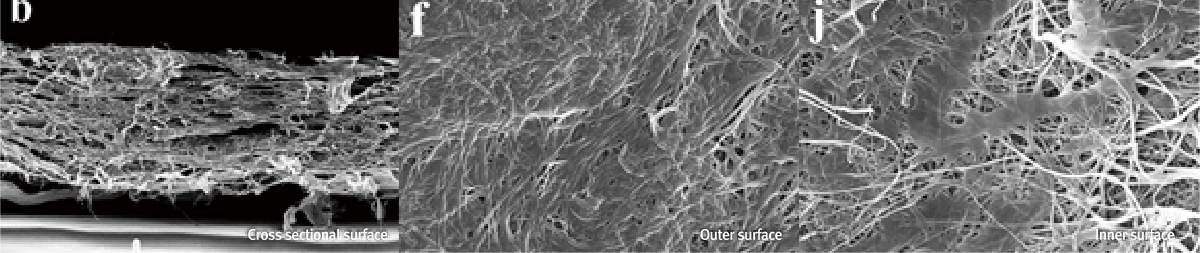
Resorbable Collagen Membrane with MedPark's Crosslinking Technology
-
Keywords
BiocompatibilityStable Decomposition PeriodSpace Maintenance
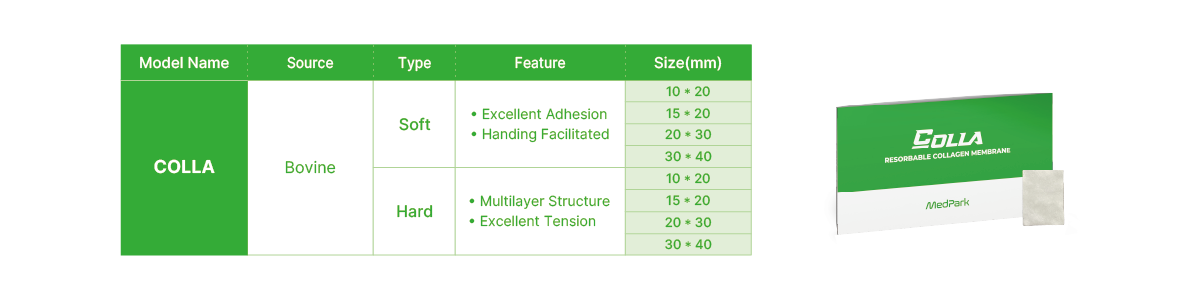
Technical Data
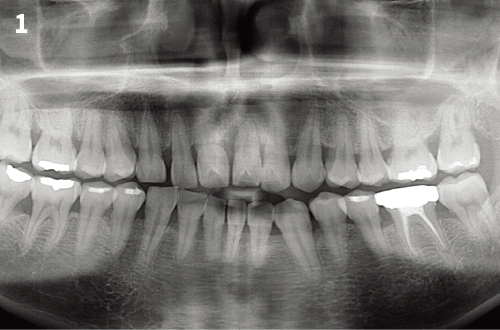
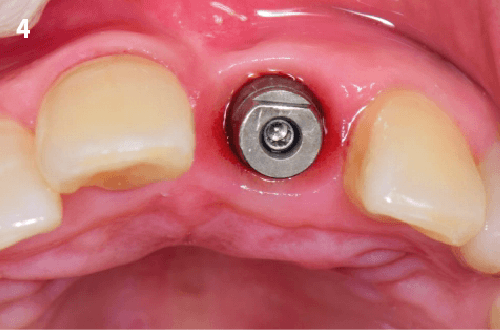
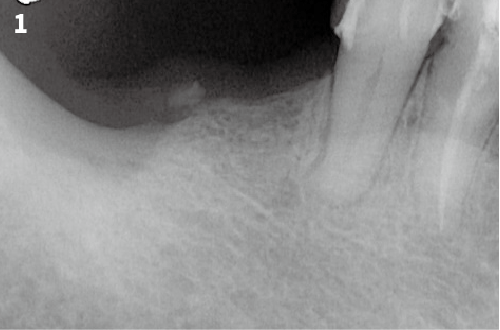
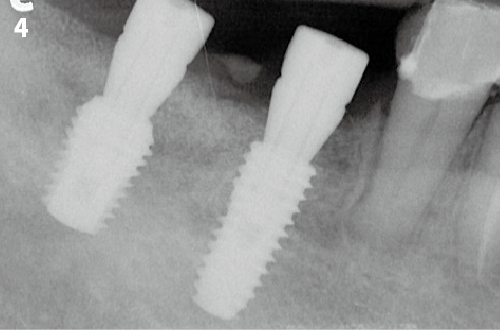
Immediate Implant Placement
Vertical Bone Augmentation
-
Intended Use
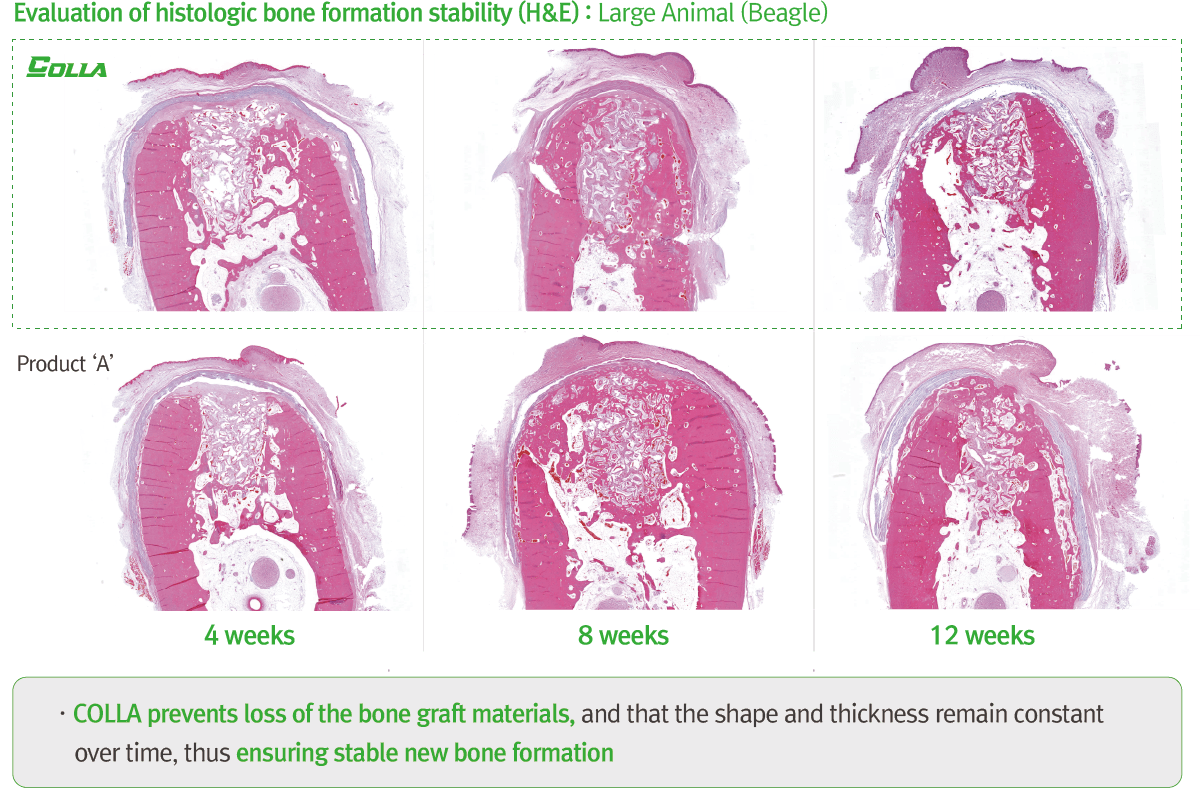
The main function of this product is to block the graft implanted in the surgical site and to prevent the invasion of foreign objects. This barrier prevents graft loss, and promotes bone conduction and bone formation during the degradation process, as well. Therefore, it can be applied in the following cases.
· Periodontal/infra bony defects
· Guided bone regeneration (GBR) procedures
· Ridge augmentation
· Sinus lifts
· Extraction sites
-
Preoperative Preparation
1. Deliver a double packed product to the operating room while keeping it sterilized.
2. Never use if the outer package or sterilized inner package is found either opened or damaged.
3. Never use if any foreign materials are found inside the sterile packaging.
4. The clinician must read Directions for USE before the surgery, and completely understand its characteristics and usage methods.
-
Directions for Use
1. Incise the gingiva in the surgical area to expose the periosteum.
2. Completely remove the granulation tissue, inflammatory tissue and other soft tissue in the binding site.
3. Check the type and size of the product as shown on packaging/labels to prepare the proper product.
※ COLLA is identical on both front and back sides. It can be used without any distinction.
4. Depending on the size of the surgical site, the selected product may need to be cut to a suitable size with sterile surgical scissors.
※ It is recommended to cut at least 2mm larger than the surgical area to cover the entire area.
※ If necessary, this membrane can be moistened for flexibility.
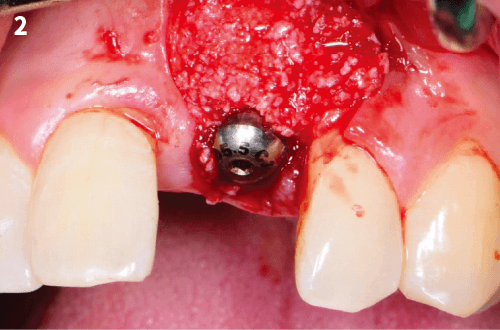
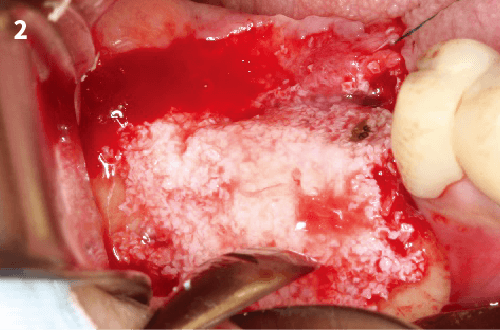
5. After implanting the bone graft, cover it with COLLA and gently press it.
※ The defect should not be overfilled.
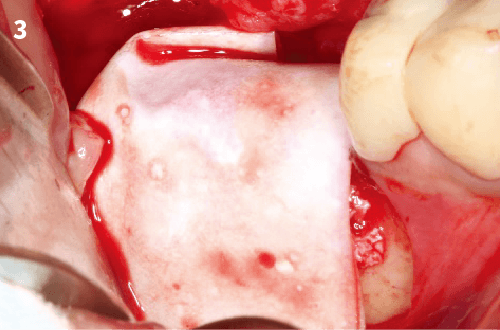
6. The membrane should overlap the walls of the defect by at least 2mm to allow complete bone contact and to prevent gingival connective tissue invasion below the material.
7. The mucoperiosteal flap is sutured over the collagen membrane with non-absorbable surgical suture.
※ At your discretion, additional suture work may be needed. -
Storage and Expiration date
This product is supplied in a sterile container. Store as it is at room temperature(1℃ to 30℃) in the shade before use. If the sterile packaging is damaged or opened, the product must not be used. The contents of the Tyvek pouch are designed for si ngle use only. Discard any unused material after opening.
Expiration date is 3(three) years from date of manufacture. Do not use and re-sterilize products that have expired. -
Precautions
1. Handling Precautions
1) This product shall not be reused or re-sterilized as a sterilized disposable medical device.
2) This product shall be used in sterile environments with sterile surgical instruments.
3) Do not use the product for any purpose other than GBR(Guided Bone Regeneration) case, or modify the product for other uses.
4) The surgical instruments must be cleaned and sterilized before being used.
5) Immoderate GBR(Guided Bone Regeneration) results in the exposure of the alveolar bone, thus, leading to failure of bone augmentation due to external infection. Therefore, both clinician and patient should be careful to avoid maximum exposure and the exposed area should be regularly screened and treated by clinicians.
6) If the product is bent or trimmed excessively, it may be damaged.
7) COLLA must be kept at 1 to 30℃. 8) Before use, check the product's precautions and instructions. 9) This product should only be used by trained dentists or a oral surgeons.
2. Contraindications
Surgery should be avoided in patients with contraindications, including :
1) Patients with known allergy to collagen of bovine origin.
2) Patients with advanced lesions with a significant reduction in residual alveolar bone volume.
3) Patients with lesions difficult to secure space, such as horizontal bone defect.
4) Patients with progressive lesions across multiple teeth that require multiple, continuous use of the product.
5) Uncontrollable diabetic patients, excessive smoking or alcoholism.
3. Side effects
1) As this product is based on bovine-derived typeⅠcollagen, an allergic reaction may occur.
2) This product is for one of the periodontal surgeries. Periodontal surgeries can cause gum distortion, deadlock and absorption of the root, inflammation, gingival changes, and complications due to anesthetics.
3) Depending on the severity and type of complications, the clinician may choose to remove the barrier and perform additional treatments.
4. Others
1) One patient can be implanted with a maximum of one 30x40(mm) size COLLA during one procedure and their lifespan.
2) It is recommended to use the size corresponding to the defect within the maximum allowable dosage. The size of the product can be determined at the discretion of the dentist.
Manufacturer
MedPark |24, Nakdong-daero 1570 beon-gil, Buk-gu, Busan, Republic of Korea | E-mail : biz@medpark.net
Sepcifications